Epithelial-Mesenchymal Transition (EMT): A Spectrum beyond Binary
Our understanding of EMT has evolved from the traditional binary view to the perspective of multiple intermediate states in between the two extreme epithelial and mesenchymal states. These intermediates states encompass a spectrum of cell states which are regulated at epigenetic, transcriptional, translational, and post-translational levels. Our lab has been focusing on defining the early events of EMT (Huang et al., J Cell Sci 2012) and has proposed the paradigm shift of the EMT concept from binary to a continuous spectrum (Huang et al., Cell Death and Disease 2013). By using big data analysis of 13,000+ cancer gene expression profiles, our group has pioneered in establishing EMT signatures and defining the EMT scoring (Tan et al., EMBO Mol Med 2014).
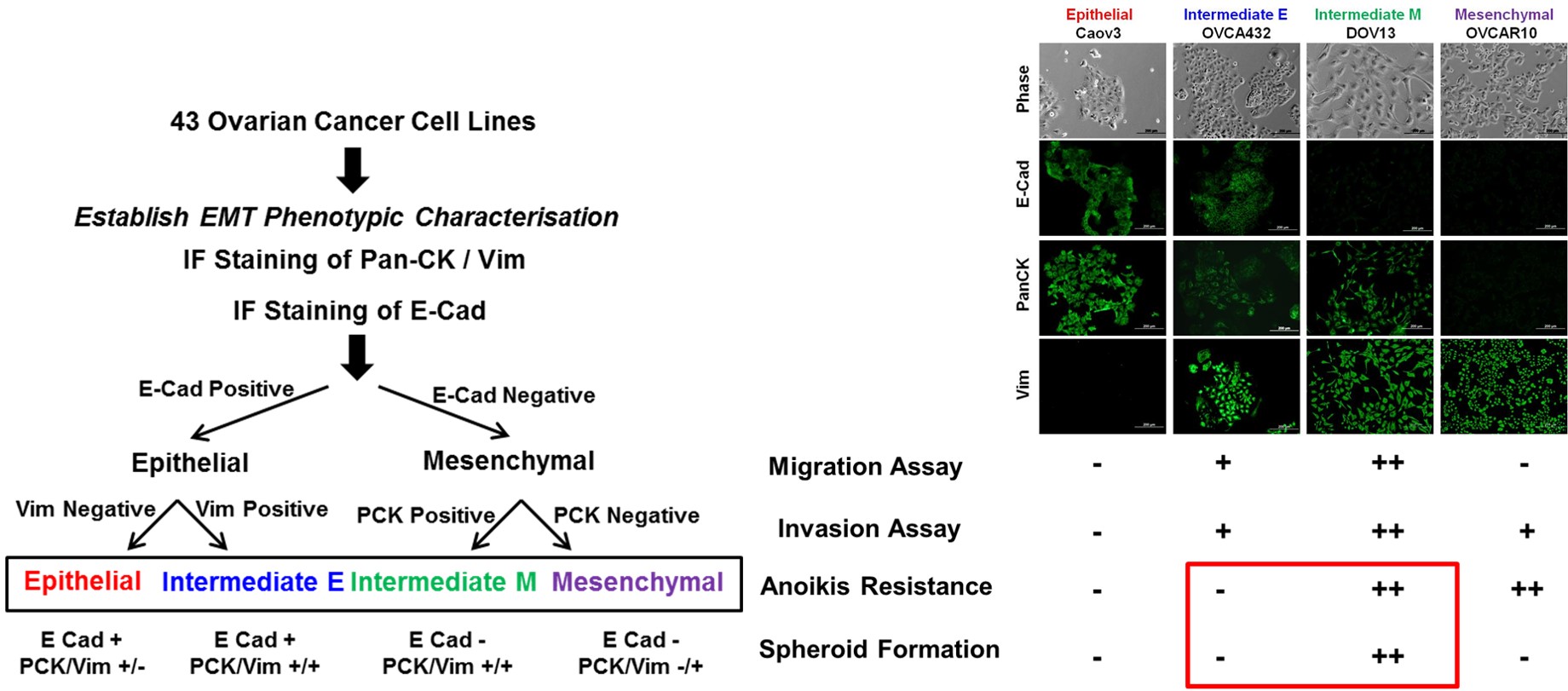
An
in vitro assessment of epithelial-mesenchyma phenotypes by the immunofluorescence (IF) staining pattern of E-cadherin, pan-cytokeratin and vimentin defines the EMT Spectrum into Epithelial (E), Intermediate Epithelial (IE), Intermediate Mesenchymal (IM) and Mesenchymal (M) states
(Huang et al., Cell Death and Disease 2013).
- GRHL2: a pioneer factor for epithelial gatekeeping
Grainyhead-like 2 (GRHL2) is one of the three mammalian orthologs of the gene grainyhead identified in Drosophila. It is a transcription factor that regulates a repertoire of epithelial-specific genes to guard the epithelial differentiation. Grhl2 plays important roles in embryonic development during neural tube closure and the morphogenesis of placenta.
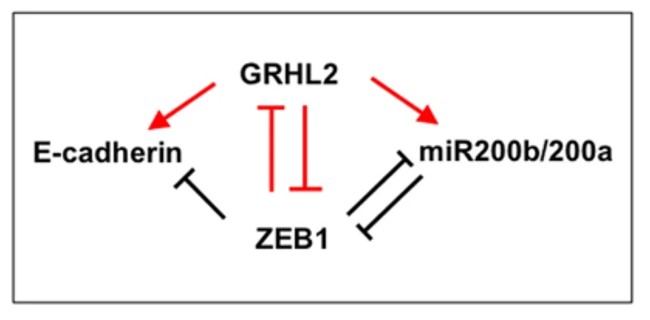
GHRL2 has been shown to suppress EMT in cancers. Previously, we reported that the loss of GRHL2 results in sequential changes in the EMT phenotype (Chung et al., Scientific Reports, 2016). GRHL2 knockdown in an Epithelial (E) cell line PEO1 leads to a phenotype switch from E to IE through an altered E-cadherin subcellular distribution and up-regulation of vimentin. In an Intermediate Epithleial (IE) cell line OVCA429, GRHL2 knockdown further switches the cells from IE to IM via the loss of junctional E-cadherin and the up-regulation of vimentin.
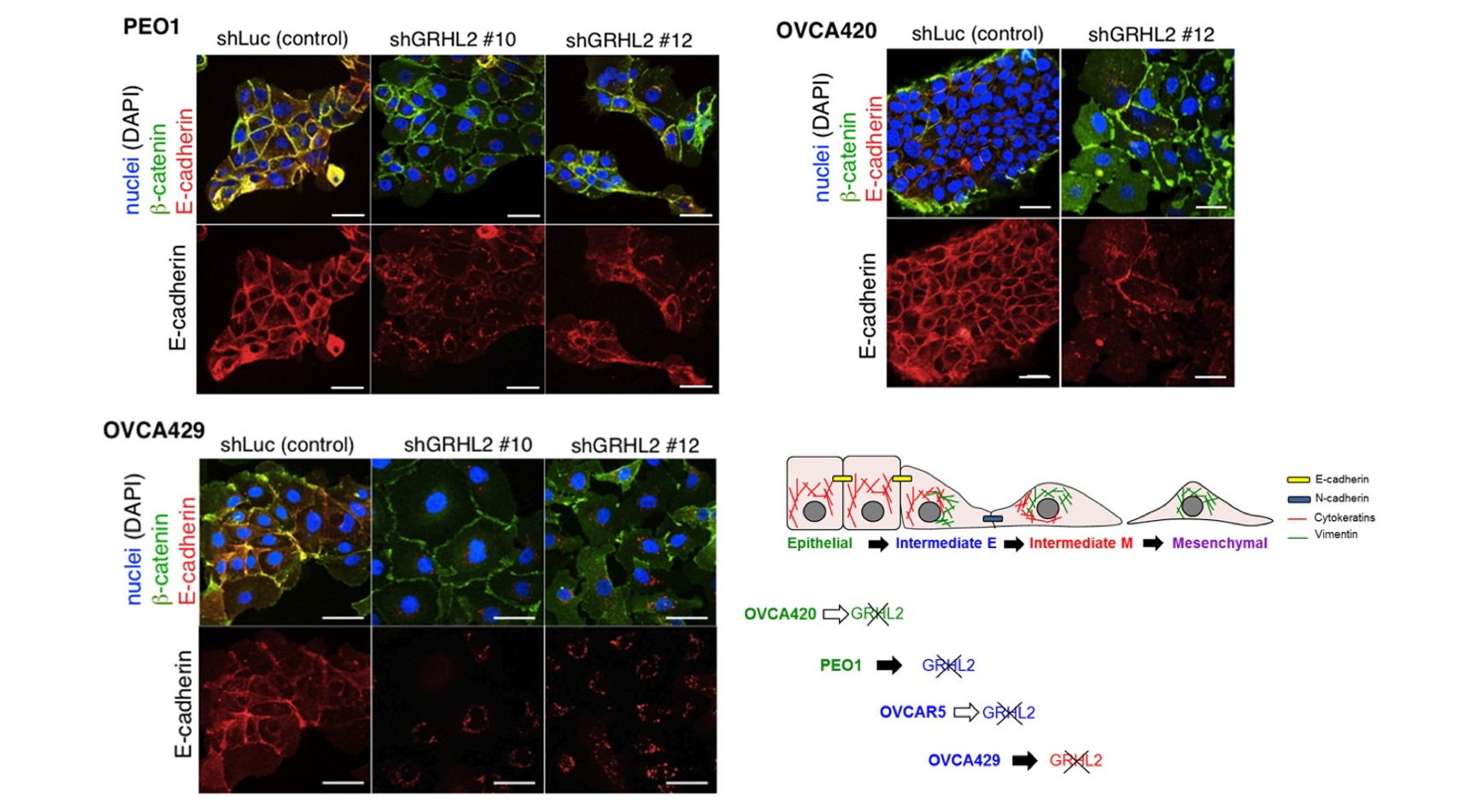
Recently, we further explored the role of GRHL2 in genome-wide epigenetic remodeling associated with EMT plasticity by integrating the analyses of DNA methylation, ChIP-sequencing of five histone marks (H3K4me1, H3K4me3, H3K27Ac, H3K27me3 and H3K9me3) and transcriptome profiling on cancer cells with different epithelial/mesenchymal states (Chung et al., Communications Biology, 2019). Our findings demonstrate that epithelial genes are subject to epigenetic control during intermediate phases of EMT/MET involving GRHL2.

- SNAI1-Driven sequential EMT changes
Snail Family Transcriptional Repressor 1 (SNAI1) is a member of the Snail superfamily of transcription factors. During cancer-associated EMT, SNAI1 enforces EMT by regulating other core transcription factors and upregulating proteins of the mesenchymal phenotype. We revealed that SNAI1 overexpression in ovarian cancers switches from a repressive regulation with an epithelial phenotype to an activation pattern with an intermediate epithelial phenotype (Sundararajan et al., Cancers, 2020).
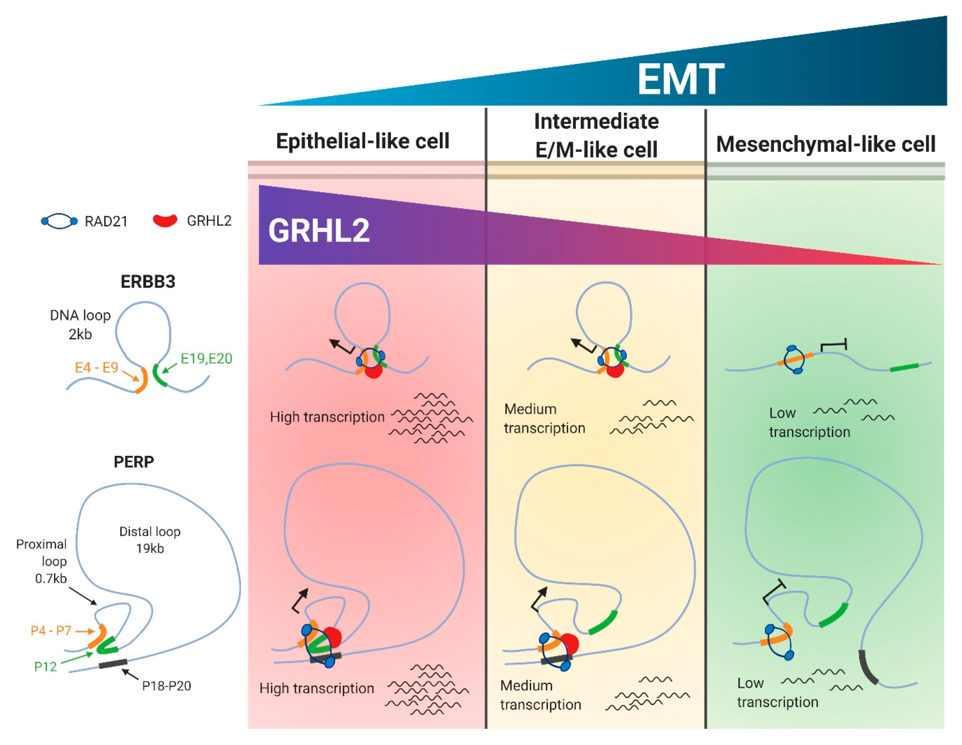
Further, we identified PERP and ERBB3 as gene targets of SNAI1(Sundararajan et al., Cancers, 2020). Detailed analysis of two candidate genes showed that selective enrichment of RAD21 and GRHL2 on the gene regulatory elements modulate their expression levels.
In the context of EMT, recent research findings have identified novel mechanisms, outlining that specific alterations in the transcriptional signature correlate with changes in chromatin conformational reorganization.
RAD21, a key structural component of the multiprotein, ring-shaped cohesin complex. Recent findings show that higher order cohesin-mediated chromatin alteration possibly regulates the spatial and temporal regulations of gene transcription. Using tetracycline-controlled transcription activation (Tet-on) system, we further shows that the association of Rad21 is likely to be dependent on the expression of GRHL2.
Results from our study suggest a proposed model where early EMT genes are subjected to differential chromatin looping alterations, and these structures are partially dependent on the expression of epithelial gatekeeper GRHL2.